Accelerating Antiviral Treatment Development for COVID-19
Written on
Chapter 1: Introduction to Antiviral Treatments
The urgent need for effective treatments for COVID-19 has led to a focus on repurposing existing medications. This strategy could significantly speed up the process of identifying viable antiviral options.
Understanding candidate treatments involves ensuring that they are both safe and effective at alleviating symptoms, shortening illness duration, or even curing the infection.
Section 1.1: Identifying Antiviral Compounds
Researchers employ various methods to pinpoint potential antiviral agents. One approach involves utilizing computer simulations to model interactions between specific compounds and viral proteins. This has driven many scientists to decode the molecular structures of the SARS-CoV-2 virus and the proteins on host cells that facilitate viral entry. These insights enable in silico drug design and screening.
Another method involves screening extensive libraries of chemicals for antiviral properties in vitro. Chemicals known to combat other viral infections are also tested against SARS-CoV-2. This process requires a suitable cell-based system that the virus can infect, alongside a reliable method for producing sufficient viral quantities for testing. Safety precautions in laboratories are crucial during these processes, especially when working with SARS-CoV-2 and Vero cells, which are derived from monkey kidneys and can yield substantial virus amounts for experiments.
Subsection 1.1.1: Toxicity Testing in Drug Development
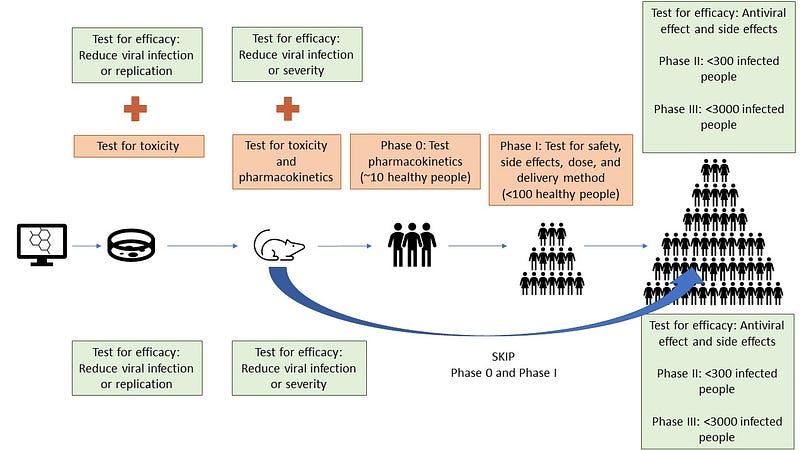
The initial phase of antiviral drug development typically involves testing in cultured cells to assess both efficacy and toxicity. For drugs that have previously been approved, only efficacy testing in these cells might be necessary. However, skipping toxicity testing does not significantly reduce the overall timeline, given that both tests can be efficiently conducted using high-throughput methods.
Drugs that demonstrate potential efficacy in vitro must then undergo toxicity assessments, often progressing to animal testing to evaluate safety further before any human trials commence.
Section 1.2: The Significance of Therapeutic Index
The ideal antiviral has a high therapeutic index, indicating a significant disparity between the dosage that causes harmful effects and the dosage that provides therapeutic benefits. A high therapeutic index ensures that the risk of adverse reactions in a large population remains low. For infections with severe mortality risks, a narrower therapeutic window might be acceptable, as the benefits of treatment can outweigh the risks.
Chapter 2: The Benefits of Repurposing Approved Drugs
Japan is moving swiftly to implement the antiviral drug remdesivir for treating COVID-19 patients, demonstrating the potential of repurposing existing treatments to address urgent health crises.
The process of testing new compounds that have never been used in humans is more complex compared to repurposing established drugs. Treatments that have already been approved can bypass initial phases of clinical testing, thus accelerating their deployment.
In a recent update, researchers discussed the ongoing efforts to identify and test existing antiviral agents for COVID-19, emphasizing the efficiency of repurposing drugs with known safety profiles.
To sum up, the rapid testing of previously approved therapies is crucial for finding effective COVID-19 treatments. This approach not only saves time but also holds promise for saving lives during the ongoing pandemic.