Vaccine Developments: Speculating on Future Timelines and Options
Written on
Chapter 1: Understanding Vaccine Types
The question "When can we expect a vaccine?" frequently arises in discussions about the COVID-19 pandemic. Initially, the timeline was uncertain, but with several vaccines now in Phase 3 trials, we are starting to gain clarity.
To begin, it’s essential to differentiate the types of vaccines currently in development. The Moderna and Pfizer-BioNTech vaccines are classified as nucleic acid vaccines. They utilize mRNA to instruct the body to create vital components of the virus, aiming to trigger an immune response. This innovative approach is relatively new, and the early results showcasing robust immune responses are remarkable, although we still cannot confirm if this response will provide adequate protection.
Another intriguing candidate is the ChAdOx vaccine, a collaboration between Oxford University and AstraZeneca. This vaccine employs a genetically modified chimpanzee adenovirus that mimics a human respiratory virus, ensuring it cannot reproduce in human cells. This modified virus is designed to produce the spike glycoprotein of the coronavirus, which is the target for both the mRNA vaccines and ChAdOx.
Here’s a video outlining human trials for the ChAdOx1 nCoV-19 vaccine:
The ChAdOx vaccine appears promising, as it utilizes a respiratory virus as a vehicle, potentially offering the immune system a "practice run" against the actual SARS-CoV-2 virus responsible for COVID-19. Generally, vaccines that closely resemble natural infections without causing disease are more effective in stimulating relevant immune responses, thus enhancing protection.
However, the data from Moderna is also compelling. Their vaccine may be sufficiently effective based on early findings. For instance, a publication in The New England Journal of Medicine illustrates that by day 36 post-vaccination, it took approximately 100 times more virus to infect cells treated with serum from vaccinated individuals compared to day one. This is noteworthy, especially since leading antiviral treatments typically only achieve a tenfold reduction in viral replication.
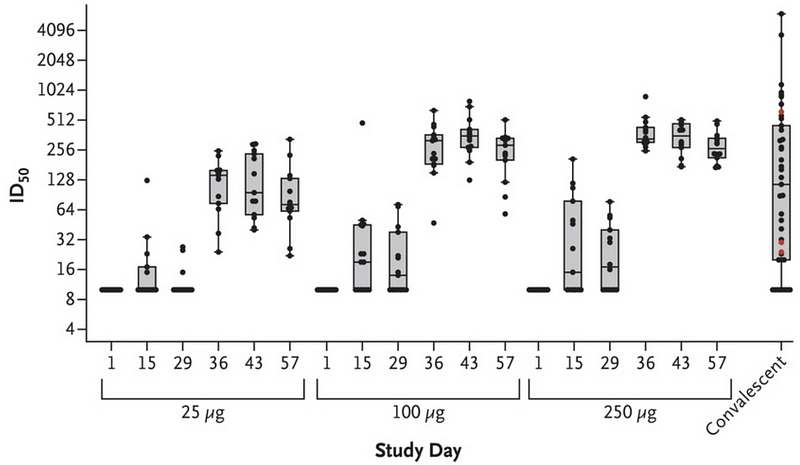
This image shows a graph corresponding to the previous discussion from The New England Journal of Medicine.
As we consider the future of vaccines, it’s crucial to evaluate the current landscape. The New York Times features a coronavirus vaccine tracker, which indicates one vaccine has received emergency approval (specifically for military use in China), while four others are progressing to Phase 3 trials, including ChAdOx.
If the current Phase 3 trials continue to yield positive results, it’s essential to project timelines. Moderna anticipates initiating its Phase 3 trials by late July, with preliminary results expected by November. While this timeline is ambitious, it sets a benchmark for what we might anticipate.
Assuming positive outcomes, we could see initial results regarding the safety and efficacy of a vaccine within four months, approximately twelve months after the virus first infected humans. This rapid development is unprecedented compared to the Hepatitis B vaccine, which took over a decade to reach the market.
Next, let’s examine the approval timeline. Once data is available, I am confident that the review and approval process for a COVID-19 vaccine will take precedence for regulatory agencies globally. I estimate that this review will require no more than one to two months, extending our timeline to six months for vaccine availability.
Companies are already manufacturing these vaccines "at-risk," which means they are producing doses without certainty of approval. Initially, I expect tens of millions of doses to be available, with production capacity rapidly increasing post-approval. If 10–50 million doses are produced in the first six months, I anticipate a jump to 100–500 million doses in the subsequent six months.
While I am not an expert in supply chain logistics, I find it reasonable to expect the first doses to be available in the U.S. market by Q1 2021, prioritized for healthcare workers and high-risk individuals. By Q2 2021, broader availability for most adults should commence, similar to the annual production of influenza vaccines. By Q3, I foresee sufficient supply for a substantial stockpile and potentially additional approved vaccines.
To summarize, here are the anticipated milestones:
- Approval of a vaccine candidate by December 2020 at the earliest.
- Availability of doses for high-risk individuals and healthcare workers by January 2021.
- General availability for most adults starting in February or March 2021, with gradual increases through May 2021.
- Sufficient supply to meet demand by June 2021.
What implications does this hold for our daily lives? I anticipate that schools will likely be able to reopen by January 2021, prioritizing teachers for early vaccination doses. However, the virus will remain present, necessitating continued social distancing and masking until at least June 2021.
This article was originally published in my daily newsletter, COVID Transmissions.
Chapter 2: Vaccine Futures and Expectations
The first video highlights the future of vaccines, discussing innovations and the potential impacts on public health.
The second video provides insights into the new COVID vaccine and variants for 2024-25, explaining how they may address ongoing challenges.