Understanding the Dry Cleaning Process: It's Not Just 'Dry'
Written on
Chapter 1: The Basics of Dry Cleaning
Have you ever wondered about the cleaning process your clothes undergo at the dry cleaners? Contrary to what the name suggests, dry cleaning isn't entirely dry. While traditional washing methods often involve soap and water, some stains—especially greasy ones—require a different approach.
When I last visited my local dry cleaners, I handed over a particularly dirty item. The attendant expertly assessed the condition, attaching a bright yellow tag before taking the garment away. I left hopeful they could restore it to its former glory. But what actually happens behind the scenes in these establishments? I often imagined tiny creatures, reminiscent of those in fairy tales, diligently cleaning clothes.

Dry Cleaning: A Misleading Name
It might surprise you to learn that when your clothes are sent for dry cleaning, they actually get quite wet. The term "dry" refers to the absence of water as a solvent. A solvent is a substance that dissolves other materials; for example, in the case of salt, water acts as the solvent.
Water is often termed the "universal solvent" due to its ability to dissolve numerous substances. Water molecules are polar, meaning they have slightly different charges at each end, which allows them to attract other polar molecules effectively.
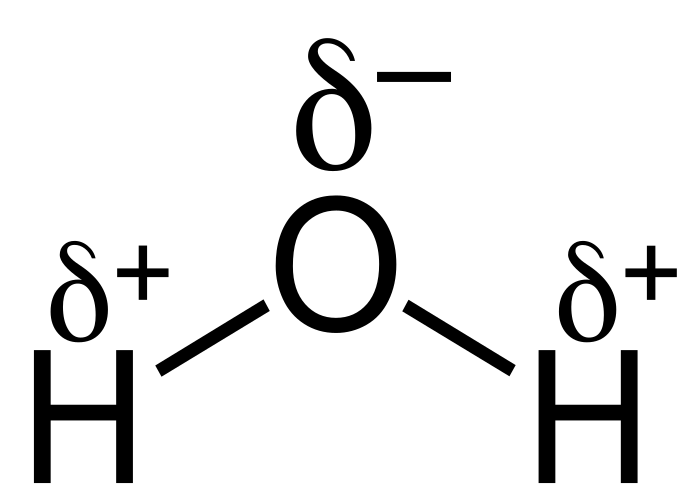
This property makes water an excellent cleaning agent, as it can bind to dirt and grime. Soap molecules enhance this process: their long hydrocarbon chains attract fats, while their charged ends interact with water, allowing dirt to be rinsed away.
However, certain stubborn stains require a non-polar solvent to lift greasy molecules out of fabric.
Commercial dry cleaning has its origins in a happy accident. In 1855, J.B. Jolly’s maid spilled kerosene on a tablecloth, resulting in a surprisingly clean cloth, thus marking the birth of commercial dry cleaning.

The Dry Cleaning Mechanism
During the dry cleaning process, garments are agitated in a solvent, much like they would be in a traditional washing machine. The clothes are placed in a perforated drum, allowing the solvent to permeate the fabric. Fresh solvent continuously circulates through the garments, while the soiled solvent is cleaned and recycled. Finally, the clothes are thoroughly dried to remove any remaining solvent through evaporation.
Historically, organic solvents like kerosene and petroleum were commonly used, although their flammability raised safety concerns. Modern dry cleaning has shifted to safer alternatives such as tetrachloroethylene (often called "perc") and other eco-friendly options like liquid silicone and carbon dioxide.
So, there are no tiny cleaning mice or miniature brushes at work. Instead, it's a straightforward application of chemistry that efficiently tackles our most stubborn stains.
This video titled "How Dry Cleaning Works" offers a visual insight into the dry cleaning process, showcasing the methods and technology involved.
The second video, "How Does Dry Cleaning Work?" delves deeper into the science behind dry cleaning, explaining the solvents and machinery used.
Want to learn more about my writing and interests? Feel free to check out my profile!