Exploring the Connection Between Alzheimer's and Type 3 Diabetes
Written on
Chapter 1: The Link Between Glucose and Alzheimer's Disease
Recent longitudinal research has explored how glucose levels in early adulthood may influence the likelihood of developing Alzheimer’s disease in later years. The study analyzed data from nearly 5,000 participants aged 35 to 50 over a span of 38 years, aiming to find correlations between metabolic markers and the onset of Alzheimer’s.
Individuals exhibiting elevated blood glucose levels during middle adulthood faced a 14.5% increased risk of Alzheimer’s disease later in life. This raises an important question: what is the relationship between compromised glucose metabolism and cognitive decline?
This paragraph will result in an indented block of text, typically used for quoting other text.
Section 1.1: The Role of Glucose in Brain Function
The brain primarily relies on glucose as its energy source, although it can also utilize ketones in fasting states. Elevated blood sugar levels hinder glucose uptake in the brain, positioning diabetes and obesity as significant risk factors for dementia.
A gene known as ApoE4 is closely linked to a heightened risk of developing Alzheimer’s. Individuals with one mutated copy of this gene double their risk, while those with two mutated copies increase their risk tenfold. Young adults carrying one copy of the ApoE4 variant display abnormally low glucose levels in brain regions commonly affected by Alzheimer’s, including the posterior cingulate, parietal, temporal, and prefrontal cortex.
Subsection 1.1.1: Insulin Regulation and Alzheimer’s

Evidence of insulin resistance in the brain has led to Alzheimer’s being referred to as “type 3 diabetes.” A recent study by Japanese researchers contributed to this understanding, suggesting that amyloid beta (Aβ), a protein often linked to neurodegeneration, may influence insulin secretion.
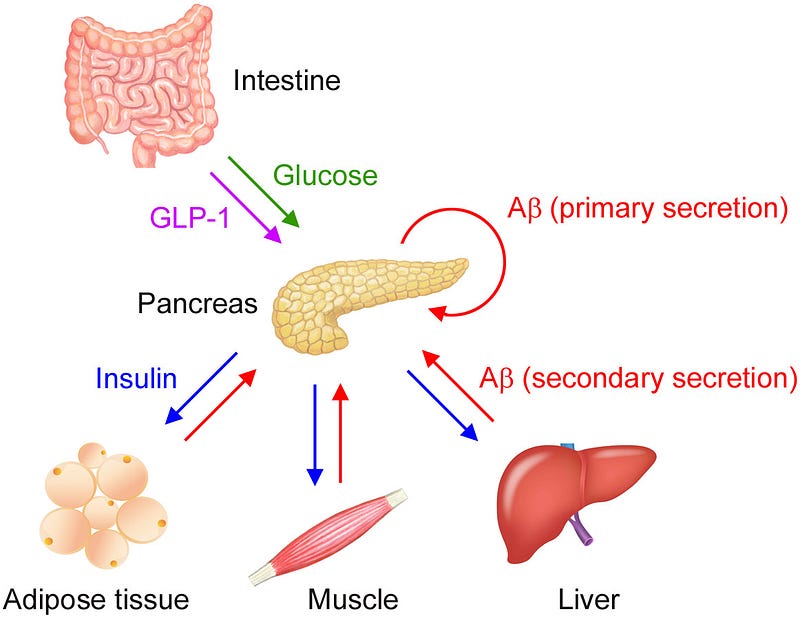
Traditionally, Aβ levels in plasma are believed to indicate brain pathology; however, the research showed that glucose- and insulin-sensitive tissues—such as the pancreas, adipose tissue, skeletal muscles, and liver—also secrete Aβ, which can inhibit insulin secretion from pancreatic beta cells.
After a meal, as blood glucose levels rise, the pancreas releases insulin to promote glucose uptake, thereby lowering blood sugar levels. Interestingly, the pancreas also releases Aβ in response to glucose stimulation, a process referred to as “primary secretion.” Insulin then circulates, binding to peripheral tissues and prompting them to secrete Aβ in a manner known as “secondary secretion.”
Section 1.2: Understanding Amyloid Beta and Its Effects
Source: Shigemori K, Nomura S, Umeda T, Takeda S, Tomiyama T. Peripheral Aβ acts as a negative modulator of insulin secretion. Proceedings of the National Academy of Sciences (2022). doi:10.1073/pnas.2117723119
While glucose is essential for insulin release, plasma Aβ may help to regulate this process. Aβ secreted from pancreatic islet cells adjusts insulin release in an autocrine fashion, while Aβ from insulin-sensitive organs inhibits insulin secretion in an endocrine manner. This dual regulation of insulin secretion aids in maintaining stable blood sugar levels.
Aβ produced by peripheral tissues is believed to function as an organokine, an endocrine factor that enables communication between organ systems, contributing to glucose and insulin regulation. In response to insulin, muscle, liver, and fat tissues release their own organokines, termed myokines, hepatokines, and adipokines, respectively.
Over a decade ago, a research team identified the APP gene as a key player in regulating insulin secretion from pancreatic islets. They reported that the absence of APP in mice resulted in increased insulin secretion in response to glucose, although the underlying mechanism was previously unclear.
We now understand that mice lacking APP show heightened insulin secretion because Aβ derived from APP acts as a negative regulator of insulin secretion. This connection hints at a relationship between Alzheimer’s and diabetes.
Chapter 2: Amyloid Beta and Diabetes—Exploring the Connections
The first video titled "Mayo Clinic Minute: Is Alzheimer's Type 3 diabetes?" delves into the relationship between Alzheimer's disease and glucose metabolism, offering insights into this intriguing connection.
The second video, "Why is Alzheimer's called Type 3 diabetes? The Brain Docs," explores the scientific basis for the classification of Alzheimer's disease as a form of diabetes, shedding light on the mechanisms at play.
How Does Amyloid Beta Accumulate in the Brain?
Aβ can be found in both the brain and plasma. For Aβ in the brain to enter the bloodstream, it must cross the blood-brain barrier (BBB) via a receptor known as low-density lipoprotein receptor-related protein 1 (LRP1). Conversely, the transport of Aβ from the bloodstream into the brain is mediated by the receptor for advanced glycation end products (RAGE).
High blood glucose and insulin levels can lead to persistent Aβ production in plasma, disrupting the balance between brain Aβ and peripheral Aβ by hindering Aβ efflux from the brain. In essence, chronically elevated Aβ levels in the bloodstream could impair the brain's ability to expel Aβ. At the same time, high circulating insulin levels can cross the blood-brain barrier, interfering with Aβ degradation in the brain by competing for insulin-degrading enzymes.
Consequently, Aβ levels in the brain may rise due to reduced excretion into the bloodstream and inadequate enzymatic breakdown. Elevated Aβ levels may begin to aggregate into soluble oligomers, which can lead to cognitive decline and trigger the cascade of events resulting in Alzheimer’s disease.
Researchers suggest that plasma Aβ levels could serve as a diagnostic biomarker for Alzheimer’s disease, but only in fasting conditions, as these levels fluctuate rapidly with food intake.